Robust Network of Global Facilities
Assuring Local Clinical Trial Success
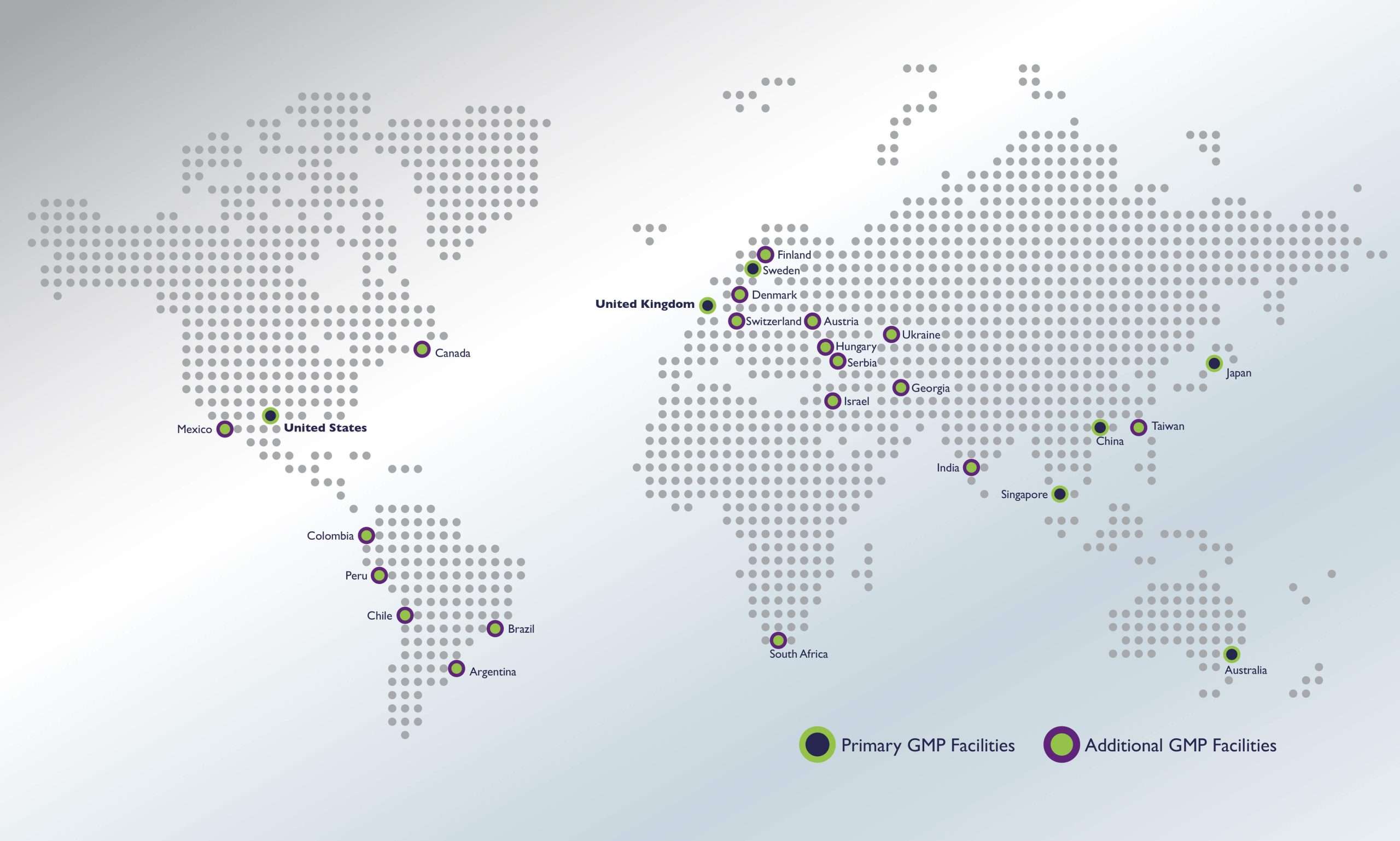
Our Global GMP Footprint
Our GMP warehouses are supported by a network of 35+ audited, GMP-certified depots, assuring the local success of our clients’ global trials.
All facilities within the network are strategically positioned near airports and other key shipping resources, allowing us to efficiently distribute clinical trial materials to serve complex, global clinical trials.
- List of Facilities
- Primary
- United Kingom
- United States
- Secondary
- Austria
- Switzerland
- Tertiary
- Japan
- Taiwan
- Primary
Our GMP Facilities
Headquarters and USA GMP Facility
Packaging and Labeling Services
- Secondary packaging, kitting & labeling, including cold chain
- Wallet packaging / rotary wallet press
- Aluminum foil pouch sealing
- In-house label printing services
- In-house blinding and randomization services
Controlled and Monitored Temperature Storage
- 80,000 sq ft. state-of-the-art facilities
- ERP with integrated inventory management system
- Ambient (15°C to 25°C), refrigerated (2°C to 8°C), frozen (-20°C), and Ultra Low (-70°C) storage conditions
- Controlled substance handling and vault storage (Schedules I through V)
- Clinical trial returns and destruction services
Approvals / Licenses
- State of Texas:
- Wholesale Distributor Prescription Drugs
- Device Manufacturer
- Drug Enforcement Agency (DEA):
- Importer Registration Certificate – Schedules I through V
- Exporter Registration Certificate – Schedules II through V
- Manufacturer Registration Certificate – Schedules I through V
- Distributor Registration Certificate – Schedules I through V
1500 Business Park Drive
Unit B
Bastrop, TX, 78602, USA
UK GMP Facility
CalCog’s UK GMP facility is strategically located near the Dartford tunnel with convenient access to London Heathrow Airport and the EU
Packaging and Labeling Services
- Secondary packaging, kitting & labeling, including cold chain
- In-house label printing services
- Qualified Person (QP) services
Controlled and Monitored Temperature Storage
- 30,000 sq ft. state-of-the-art facility
- ERP with integrated inventory management system
- Ambient (15°C to 25°C), Refrigerated (2°C to 8°C), and Frozen (-20°C) storage conditions
- Controlled Substance Handling (Schedules 2 through 5)
- Clinical trial returns and destruction services
Approvals / Licences
- Medicines & Healthcare products Regulatory Agency (MHRA):
- Wholesale Distribution Authorisation – WDA(H)
- Manufacturer’s Authorisation – Investigational Medicinal Products – MIA(IMP)
- Manufacturer’s/Importer’s Licence– MIA
- Manufacturer’s “Specials” Licence – MS
-
Home Office – UK Controlled Drug Licence:
- Schedule 2 – possess and supply
- Schedule 3 – possess and supply
- Schedule 4 (part 1 and part 2) – possess and supply
- Schedule 5 – supply
Unit 8 Masthead, Capstan Court
Crossways Business Park
Dartford, DA2 6QG, UK
Do You Have Clinical Trial Storage & Distribution Needs?
CalCog’s team of warehousing and clinical trial supply chain experts are here to help.