Secondary Packaging & Labeling Capabilities
Meeting the Rigors of Your Clinical Trial
No matter your trial’s size, phase, or design, CalCog’s secondary packaging and labeling capabilities are here to support your clinical supply needs.
For more than nearly three decades, CalCog has provided innovators and contract research organizations (CROs) with efficient and adaptive solutions, reducing risk in the R&D supply chain and delivering investigational medication to patients faster.
CalCog Clinical Packaging & Labeling Services
We work behind the scenes assuring quality, on-time, and compliant secondary packaging and labeling, allowing your clinical trial supply chain to operate smoothly so you can focus on what matters most, delivering your medical innovations to patients in need.
Secondary Packaging & Labeling Services At-a-Glance
- Handling of diverse materials from small molecule therapeutics to biologics
- Cold chain packaging and labeling within dedicated refrigerated and frozen GMP areas
- Schedule I through V controlled substance packaging and labeling
- Specialized capabilities like blister strip wallet card creation, metered dose inhaler (MDI) assembly, foil pouch heat sealing
- Complex, multi-component kit packaging
- Bulk labeling & packing for depot shipments
- Rework & relabeling of finished goods
- In-house label printing services
- In-house randomization services
- Just-in-time labeling
- Concept to Dispatch in 30 Days program
- Adaptive scheduling offers flexibility to respond to supply chain delays
Our Secondary Packaging & Labeling Capabilities
The following offers a more detailed look at our secondary packaging and labeling operational capabilities. In addition to our clinical packaging and labeling operations, we offer comprehensive clinical packaging and label design services.
Cold Chain Packaging & Labeling
- Packaging and labeling solutions for temperature-sensitive drug product, maintaining adherence to their labeled conditions
- Secondary packaging and labeling within a dedicated refrigerated GMP area, and at our US headquarters, within a frozen environment (-25°C to -15°C)
- Our design experts select each label and carton for optimal refrigerated and frozen material adherence
Multi-Component Ancillary Supply Kits
- Kitting capabilities from a single-visit ancillary kit containing a sterile needle and syringe to pharmacokinetic (PK) sample kits containing several sizes of cryovials, collection tubes, and alcohol swabs
- Kits are processed within our GMP areas; all supplies are counted, inspected, and reconciled to ensure suitability for use at clinical trial sites
Multiple-Product Kitting
- Labeling and assembly of patient packs containing different drug products
- Kits are processed within our GMP areas; all supplies are counted, inspected, and reconciled to ensure blinding, traceability, and accuracy is maintained and documented
Wallet Card Manufacturing
- An ideal solution to perform blinding of primary packaged investigational product with a comparator
- Bespoke wallet cards can accommodate single or multiple blister strips
- Printed wallet options can include diagrams and information to enhance patient compliance
- Processes accommodate solid-dose medication, including hard or soft gel capsules
- Child-resistant regulations compliance available
- Thorough in-process quality control inspections ensure the integrity of the finished product
Aluminum Foil Pouch Sealing
- An ideal solution for secure packaging of medical devices, such as inhalers or nasal sprays, providing an additional vapor barrier for humidity-sensitive products
Packaging of Light-sensitive Products
- Production suites outfitted with amber lighting to accommodate light-sensitive products
Metered Dose Inhaler (MDI) Blinding & Assembly
- Removal of the canister containing active product, then replacement with a placebo-filled canister
- Removal of manufacturer ink markings
- Masking of identifying features
- Replacing the dose indicator on specific designs
Finished Good Rework & Relabeling
- Expiry update operations
- Replacement of individual drug products or ancillary items
- Relabeling of clinical supplies to reassign to alternative study protocols
Just-in-time Labeling
- An ideal approach for studies requiring partial customization of clinical trial kits at the time of distribution with no disruption to timelines.
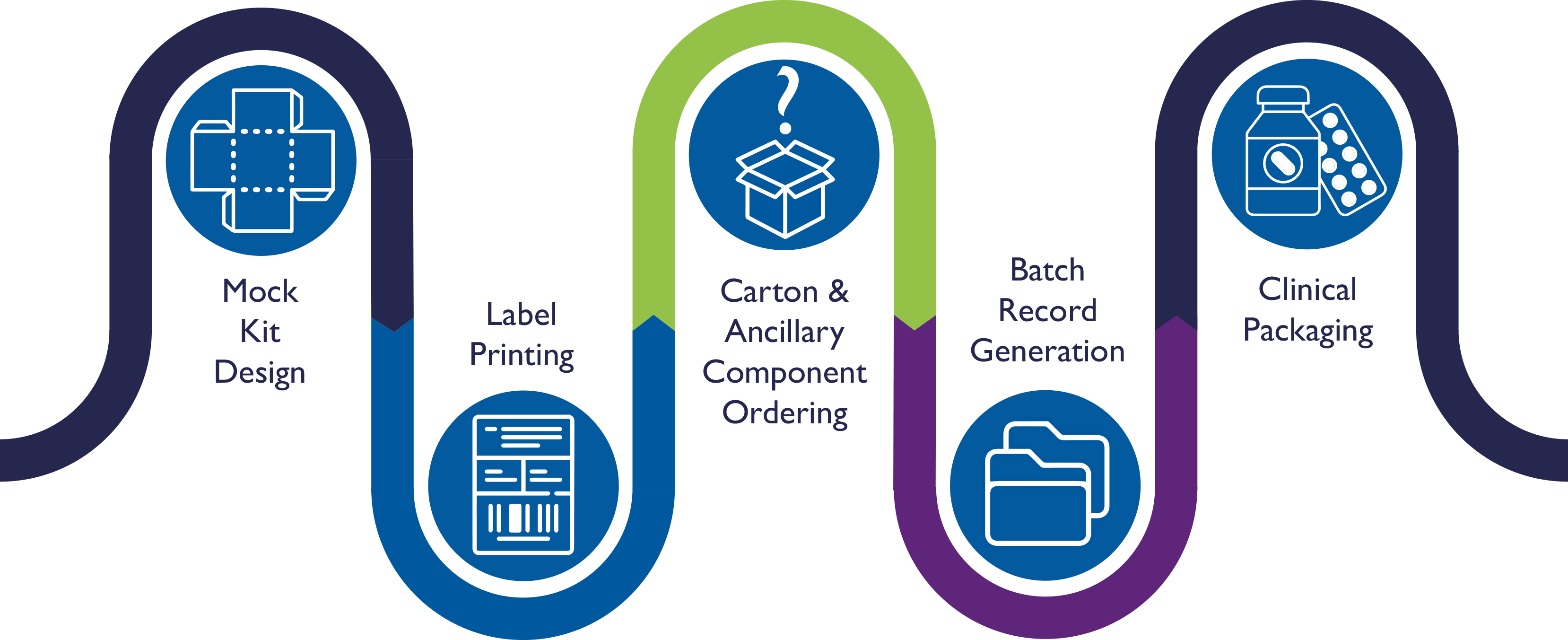
Our Consultative Approach to Secondary Packaging & Labeling
CalCog’s clients benefit from collaborative, white glove service throughout the secondary packaging and label design and packaging execution process.
Mock Kit Design
CalCog’s packaging and design services team develops a mock kit to help determine the optimal kit components, ensure correct component design and placement, and capture photos for the packaging and labeling batch record
Label Printing
CalCog’s label services team prints the required labels using fully qualified thermal transfer printers to ensure the label content remains intact in storage ranging from +25°C to -80°C
Carton & Ancillary Component Ordering
CalCog’s pre-production team orders the required components and tracks the status to ensure the production timeline moves forward without delay
Batch Record Generation
CalCog’s pre-production team leverages mock kits to create a bespoke labeling batch record and to clearly illustrate the clinical packaging and labeling requirements of every protocol we are to execute. In addition to operational excellence, the secondary packaging and labeling batch records for clinical trial supplies and Expanded Access Programs (EAPs) adhere to the applicable MHRA, EMA, and FDA GMP regulations
Clinical Packaging
CalCog’s experienced production team follows the approved Batch Record and our robust packaging and labeling standard operating procedures to govern and execute production activities carefully
Our Consultative, Collaborative Approach
“I’ve found that the journey to the clinic is most often fluid, fast-paced, and complex. Our team is really good at navigating these challenges, enabling our clients to get to the clinic quickly through solutions like our Concept to Dispatch in 30 Days program and managing inevitable scheduling changes.
We also offer solutions to fit specific needs, such as just-in-time labeling.”
Kerry Myers
Chief Business Officer, CalCog
29
Years Supporting Clinical Programs
200+
Active Programs
1.5M+
Units Labeled Annually
6
Continents with Active Programs
Responsive Clinical Trial Packaging and Labeling & Regulatory Compliance Expertise
Regulatory Compliance Expertise
Our experienced team understands the ever-changing global regulations and guidelines to facilitate the success of your program. Our knowledgeable quality assurance, quality control, and regulatory affairs teams are experts at bringing investigational products and compounds to market, working within regulatory guidelines to ensure the smooth delivery of your clinical supplies.
Our global quality management system (QMS) strictly adheres to the current best practices:
- Good manufacturing practices (GMP)
- Good distribution practices (GDP)
- Good clinical practices (GCP)
Additionally, we ensure compliance by following robust standard operating practices developed using FDA, MHRA, EMA, and ICH guidances.
Adaptive Execution
The journey to the clinic can be fluid, fast-paced, and complex. CalCog is here to navigate the challenges.

Get to the Clinic Quickly
If you are working under a pressured timeline, our Concept to Dispatch in 30 Days program may be just what you need.
Scheduling Changes
While staying on schedule is essential, some delays cannot be helped. CalCog openly communicates with you, and our adaptive systems go to work to accommodate the change.

Just-in-time Labeling
Just-in-time labeling capabilities offer flexibility without disrupting distribution timelines if partial customization of kits at the time of distribution is needed.
Do You Have Clinical Trial Packaging & Labeling Needs?
CalCog’s full suite of clinical packaging and labeling capabilities, regulatory expertise, and nimble approaches are here to help.

